Page 84 - ICSE Chemistry 8
P. 84
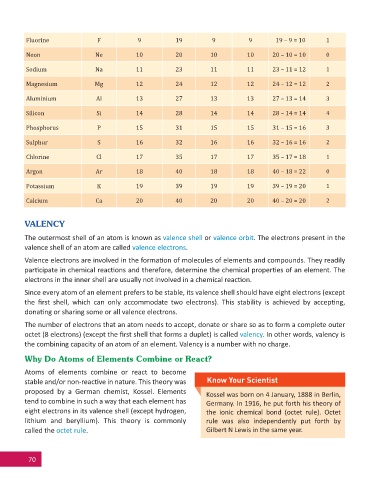
Fluorine F 9 19 9 9 19 – 9 = 10 1
Neon Ne 10 20 10 10 20 – 10 = 10 0
Sodium Na 11 23 11 11 23 – 11 = 12 1
Magnesium Mg 12 24 12 12 24 – 12 = 12 2
Aluminium Al 13 27 13 13 27 – 13 = 14 3
Silicon Si 14 28 14 14 28 – 14 = 14 4
Phosphorus P 15 31 15 15 31 – 15 = 16 3
Sulphur S 16 32 16 16 32 – 16 = 16 2
Chlorine Cl 17 35 17 17 35 – 17 = 18 1
Argon Ar 18 40 18 18 40 – 18 = 22 0
Potassium K 19 39 19 19 39 – 19 = 20 1
Calcium Ca 20 40 20 20 40 – 20 = 20 2
VALENCY
The outermost shell of an atom is known as valence shell or valence orbit. The electrons present in the
valence shell of an atom are called valence electrons.
Valence electrons are involved in the formaƟ on of molecules of elements and compounds. They readily
parƟ cipate in chemical reacƟ ons and therefore, determine the chemical properƟ es of an element. The
electrons in the inner shell are usually not involved in a chemical reacƟ on.
Since every atom of an element prefers to be stable, its valence shell should have eight electrons (except
the fi rst shell, which can only accommodate two electrons). This stability is achieved by accepƟ ng,
donaƟ ng or sharing some or all valence electrons.
The number of electrons that an atom needs to accept, donate or share so as to form a complete outer
octet (8 electrons) (except the fi rst shell that forms a duplet) is called valency. In other words, valency is
the combining capacity of an atom of an element. Valency is a number with no charge.
Why Do Atoms of Elements Combine or React?
Atoms of elements combine or react to become
stable and/or non-reacƟ ve in nature. This theory was Know Your Scientist
proposed by a German chemist, Kossel. Elements
Kossel was born on 4 January, 1888 in Berlin,
tend to combine in such a way that each element has Germany. In 1916, he put forth his theory of
eight electrons in its valence shell (except hydrogen, the ionic chemical bond (octet rule). Octet
lithium and beryllium). This theory is commonly rule was also independently put forth by
called the octet rule. Gilbert N Lewis in the same year.
70