Page 88 - ICSE Chemistry 8
P. 88
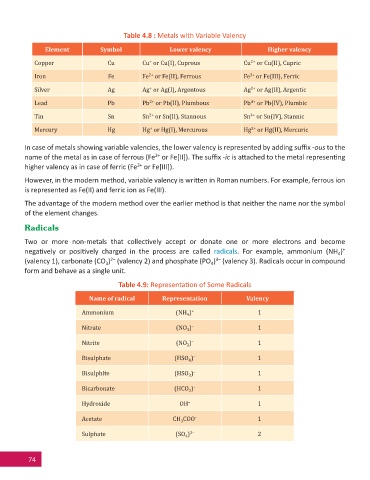
Table 4.8 : Metals with Variable Valency
Element Symbol Lower valency Higher valency
+
2+
Copper Cu Cu or Cu(I), Cuprous Cu or Cu(II), Cupric
3+
Iron Fe Fe or Fe(II), Ferrous Fe or Fe(III), Ferric
2+
2+
+
Silver Ag Ag or Ag(I), Argentous Ag or Ag(II), Argentic
Lead Pb Pb or Pb(II), Plumbous Pb or Pb(IV), Plumbic
2+
4+
Tin Sn Sn or Sn(II), Stannous Sn or Sn(IV), Stannic
2+
4+
2+
+
Mercury Hg Hg or Hg(I), Mercurous Hg or Hg(II), Mercuric
In case of metals showing variable valencies, the lower valency is represented by adding suffi x -ous to the
2+
name of the metal as in case of ferrous (Fe or Fe[II]). The suffi x -ic is a ached to the metal represen ng
3+
higher valency as in case of ferric (Fe or Fe[III]).
However, in the modern method, variable valency is wri en in Roman numbers. For example, ferrous ion
is represented as Fe(II) and ferric ion as Fe(III).
The advantage of the modern method over the earlier method is that neither the name nor the symbol
of the element changes.
Radicals
Two or more non-metals that collec vely accept or donate one or more electrons and become
nega vely or posi vely charged in the process are called radicals. For example, ammonium (NH ) +
4
3–
2–
(valency 1), carbonate (CO ) (valency 2) and phosphate (PO ) (valency 3). Radicals occur in compound
3
4
form and behave as a single unit.
Table 4.9: Representa on of Some Radicals
Name of radical Representation Valency
Ammonium (NH ) + 1
4
Nitrate (NO ) – 1
3
Nitrite (NO ) – 1
2
Bisulphate (HSO ) – 1
4
Bisulphite (HSO ) – 1
3
Bicarbonate (HCO ) – 1
3
Hydroxide OH – 1
Acetate CH COO – 1
3
Sulphate (SO ) 2– 2
4
74