Page 83 - ICSE Chemistry 8
P. 83
5
4nn
4 5nn 6 6nn
2nn 3p 4p
2
4p
3p
5p
1 5p
1pp
2p
2p
Hydrogen (1) Helium (2) Lithium (3) Beryllium (4) Boron (5)
6n 7n 8n 10n 10n
6p 7p 8p 9p 10p
Carbon (6) Nitrogen (7) Oxygen (8) Fluorine (9) Neon (10)
12n 12n 14n 14n 16n
11p 12p 13p 14p 15p
Sodium (11) Magnesium (12) Aluminium (13) Silicon (14) Phosphorus (15)
20
1 16n6n 18n 22n 2 20n0n 20nn
20p
16p 17p 18p 19p 20p
16p
19p
Sulphur (16) Chlorine (17) Argon (18) Potassium (19) Calcium (20)
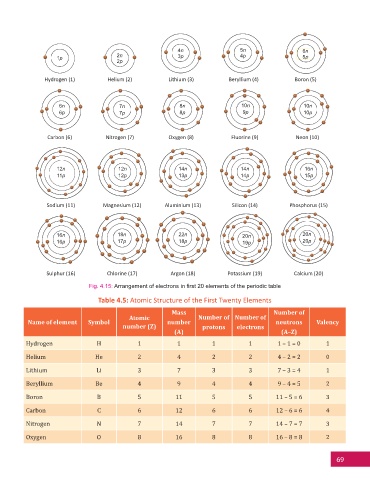
Fig. 4.15: Arrangement of electrons in fi rst 20 elements of the periodic table
Table 4.5: Atomic Structure of the First Twenty Elements
Mass Number of
Atomic Number of Number of
Name of element Symbol number neutrons Valency
number (Z) protons electrons
(A) (A–Z)
Hydrogen H 1 1 1 1 1 – 1 = 0 1
Helium He 2 4 2 2 4 – 2 = 2 0
Lithium Li 3 7 3 3 7 – 3 = 4 1
Beryllium Be 4 9 4 4 9 – 4 = 5 2
Boron B 5 11 5 5 11 – 5 = 6 3
Carbon C 6 12 6 6 12 – 6 = 6 4
Nitrogen N 7 14 7 7 14 – 7 = 7 3
Oxygen O 8 16 8 8 16 – 8 = 8 2
69