Page 82 - ICSE Chemistry 8
P. 82
Therefore, the maximum number of electrons in the fi rst four shells would be 2, 8, 18, 32.
• The outermost shell also called the valence shell cannot accommodate more than eight electrons
(octet) except in case of the fi rst shell (K shell) which cannot have more than two electrons (duplet).
The reason behind this is that the presence of eight electrons in the outermost shell makes the
atom very stable.
Let’s understand this be er with the help of an example. The atomic number of hydrogen is 1, which
means it has only 1 electron, which will occupy the fi rst shell K.
The atomic number of lithium is 3, so its fi rst two electrons will occupy the K shell and the third electron
will occupy the next shell L as the K shell cannot accommodate more than 2 electrons.
Hence, the electronic confi gura on of lithium is wri en as 2, 1.
Similarly, phosphorous with atomic number 15 will have the electronic confi gura on 2, 8, 5. This shows
that the K shell has 2 electrons, the L shell has 8 electrons and the M shell has 5 electrons.
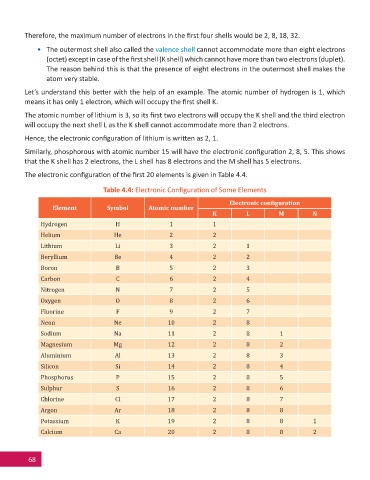
The electronic confi gura on of the fi rst 20 elements is given in Table 4.4.
Table 4.4: Electronic Confi gura on of Some Elements
Electronic conϐiguration
Element Symbol Atomic number
K L M N
Hydrogen H 1 1
Helium He 2 2
Lithium Li 3 2 1
Beryllium Be 4 2 2
Boron B 5 2 3
Carbon C 6 2 4
Nitrogen N 7 2 5
Oxygen O 8 2 6
Fluorine F 9 2 7
Neon Ne 10 2 8
Sodium Na 11 2 8 1
Magnesium Mg 12 2 8 2
Aluminium Al 13 2 8 3
Silicon Si 14 2 8 4
Phosphorus P 15 2 8 5
Sulphur S 16 2 8 6
Chlorine Cl 17 2 8 7
Argon Ar 18 2 8 8
Potassium K 19 2 8 8 1
Calcium Ca 20 2 8 8 2
68