Page 79 - ICSE Chemistry 8
P. 79
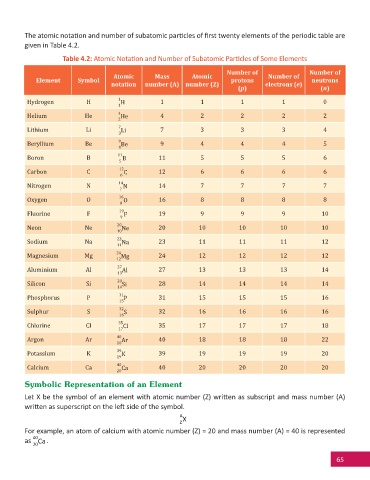
The atomic nota on and number of subatomic par cles of fi rst twenty elements of the periodic table are
given in Table 4.2.
Table 4.2: Atomic Nota on and Number of Subatomic Par cles of Some Elements
Number of Number of
Atomic Mass Atomic Number of
Element Symbol protons neutrons
notation number (A) number (Z) electrons (e)
(p) (n)
Hydrogen H 1 H 1 1 1 1 0
1
Helium He 4 He 4 2 2 2 2
2
Lithium Li 7 Li 7 3 3 3 4
3
Beryllium Be 9 Be 9 4 4 4 5
4
Boron B 11 B 11 5 5 5 6
5
Carbon C 12 C 12 6 6 6 6
6
Nitrogen N 14 N 14 7 7 7 7
7
Oxygen O 16 O 16 8 8 8 8
8
Fluorine F 19 F 19 9 9 9 10
9
Neon Ne 20 Ne 20 10 10 10 10
10
Sodium Na 23 Na 23 11 11 11 12
11
Magnesium Mg 24 Mg 24 12 12 12 12
12
Aluminium Al 27 Al 27 13 13 13 14
13
Silicon Si 28 Si 28 14 14 14 14
14
Phosphorus P 31 P 31 15 15 15 16
15
Sulphur S 32 S 32 16 16 16 16
16
Chlorine Cl 35 Cl 35 17 17 17 18
17
Argon Ar 40 Ar 40 18 18 18 22
18
Potassium K 39 K 39 19 19 19 20
19
Calcium Ca 40 Ca 40 20 20 20 20
20
Symbolic Representation of an Element
Let X be the symbol of an element with atomic number (Z) wri en as subscript and mass number (A)
wri en as superscript on the le side of the symbol.
A
Z X
For example, an atom of calcium with atomic number (Z) = 20 and mass number (A) = 40 is represented
40
as Ca.
20
65