Page 80 - ICSE Chemistry 8
P. 80
RELATIVE ATOMIC MASS
Thirst for Knowledge
Atoms are too small to be weighed. So scien sts focused
on fi nding out a standard atom and calcula ng the rela ve The average mass of one atom of an
weight of atoms of other elements by comparing it with the element is used because elements
mass of the standard atom. may have atoms of diff erent masses.
This is because the number of
Earlier hydrogen atom was chosen as the standard atom
neutrons in the nucleus may diff er
for this purpose. As me passed, the scale of atomic weight from atom to atom.
based on hydrogen was abandoned and the term atomic
weight was also replaced by another term called the rela ve atomic mass of an element.
The rela ve atomic mass of an element is the number of mes by which the average mass of one atom of
an element is heavier than 1/12th of the mass of a carbon atom (assigned atomic mass 12). It is unitless
as it is a pure ra o.
Average mass of one atom of an element
Rela ve atomic mass =
One-twel h of the mass of one atom of carbon-12
It is expressed in unifi ed atomic mass unit (and denoted as u).
Thus, the atomic mass of hydrogen atom is 1 u and that of oxygen atom is 16 u
ISOTOPES
Atoms of the same element having same atomic number but diff erent mass number are known as
14
15
isotopes. For example, N and N are the two isotopes of nitrogen. There are 7 protons and 7 electrons
7 7 14
in both the isotopes. But the fi rst atom of nitrogen ( N) has 7 neutrons and the second atom of nitrogen
15 7
( N) has 8 neutrons.
7
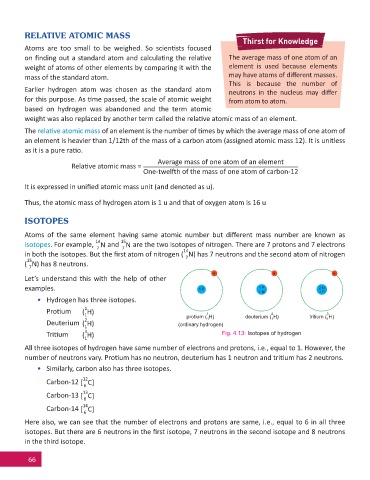
e e e
Let’s understand this with the help of other
examples. 1 P 1 P 2 N
1 N 1 P
• Hydrogen has three isotopes.
1
Pro um ( H)
1
3
2
1 protium ( H) deuterium ( H) tritium ( H)
2 1 1 1
Deuterium ( H) (ordinary hydrogen)
1
3
Tri um ( H) Fig. 4.13: Isotopes of hydrogen
1
All three isotopes of hydrogen have same number of electrons and protons, i.e., equal to 1. However, the
number of neutrons vary. Pro um has no neutron, deuterium has 1 neutron and tri um has 2 neutrons.
• Similarly, carbon also has three isotopes.
12
Carbon-12 [ C]
6
13
Carbon-13 [ C]
6
14
Carbon-14 [ C]
6
Here also, we can see that the number of electrons and protons are same, i.e., equal to 6 in all three
isotopes. But there are 6 neutrons in the fi rst isotope, 7 neutrons in the second isotope and 8 neutrons
in the third isotope.
66