Page 102 - ICSE Chemistry 8
P. 102
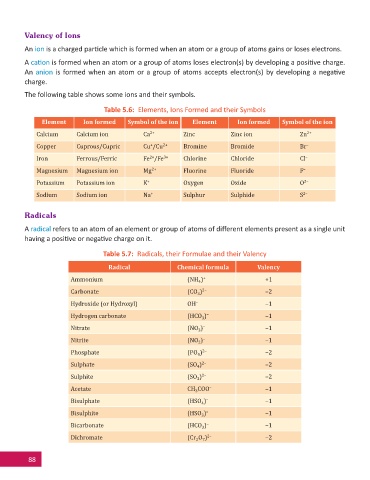
Valency of Ions
An ion is a charged par cle which is formed when an atom or a group of atoms gains or loses electrons.
A ca on is formed when an atom or a group of atoms loses electron(s) by developing a posi ve charge.
An anion is formed when an atom or a group of atoms accepts electron(s) by developing a nega ve
charge.
The following table shows some ions and their symbols.
Table 5.6: Elements, Ions Formed and their Symbols
Element Ion formed Symbol of the ion Element Ion formed Symbol of the ion
Calcium Calcium ion Ca 2+ Zinc Zinc ion Zn 2+
Copper Cuprous/Cupric Cu /Cu 2+ Bromine Bromide Br –
+
Iron Ferrous/Ferric Fe /Fe 3+ Chlorine Chloride Cl –
2+
Magnesium Magnesium ion Mg 2+ Fluorine Fluoride F –
Potassium Potassium ion K + Oxygen Oxide O 2–
Sodium Sodium ion Na + Sulphur Sulphide S 2–
Radicals
A radical refers to an atom of an element or group of atoms of diff erent elements present as a single unit
having a posi ve or nega ve charge on it.
Table 5.7: Radicals, their Formulae and their Valency
Radical Chemical formula Valency
Ammonium (NH ) + +1
4
Carbonate (CO ) 2– –2
3
Hydroxide (or Hydroxyl) OH – –1
Hydrogen carbonate (HCO ) – –1
3
Nitrate (NO ) – –1
3
Nitrite (NO ) – –1
2
Phosphate (PO ) 2– –2
4
Sulphate (SO ) 2– –2
4
Sulphite (SO ) 2– –2
3
Acetate CH COO – –1
3
Bisulphate (HSO ) – –1
4
Bisulphite (HSO ) – –1
3
Bicarbonate (HCO ) – –1
3
Dichromate (Cr O ) 2– –2
2
7
88