Page 101 - ICSE Chemistry 8
P. 101
Valency of Elements
The outermost shell of an atom is called the valence shell and the electrons present in the valence shell
are called valence electrons. Valency is usually equal to the number of electrons in the outermost shell of
the atom of that element if that number is less than 5. If the number in the outermost electrons is 5 or
more than 5, the valency is 8 minus that number. For example, oxygen has 6 electrons in the outermost
shell, its valency is 2 (8 – 6). We have learnt about monovalent, divalent and trivalent elements (valency
1, 2, and 3) in the previous chapter. In addi on to these, there also exist tetravalent and pentavalent
elements that exhibit valency 4 and 5. There are elements which also exhibit mul ple valencies and are
said to be having variable valency. The following tables show the valencies of various elements.
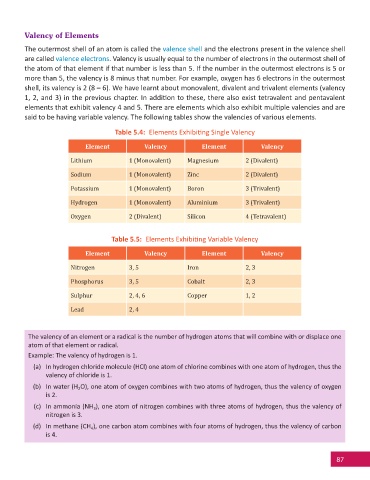
Table 5.4: Elements Exhibi ng Single Valency
Element Valency Element Valency
Lithium 1 (Monovalent) Magnesium 2 (Divalent)
Sodium 1 (Monovalent) Zinc 2 (Divalent)
Potassium 1 (Monovalent) Boron 3 (Trivalent)
Hydrogen 1 (Monovalent) Aluminium 3 (Trivalent)
Oxygen 2 (Divalent) Silicon 4 (Tetravalent)
Table 5.5: Elements Exhibi ng Variable Valency
Element Valency Element Valency
Nitrogen 3, 5 Iron 2, 3
Phosphorus 3, 5 Cobalt 2, 3
Sulphur 2, 4, 6 Copper 1, 2
Lead 2, 4
The valency of an element or a radical is the number of hydrogen atoms that will combine with or displace one
atom of that element or radical.
Example: The valency of hydrogen is 1.
(a) In hydrogen chloride molecule (HCl) one atom of chlorine combines with one atom of hydrogen, thus the
valency of chloride is 1.
(b) In water (H O), one atom of oxygen combines with two atoms of hydrogen, thus the valency of oxygen
2
is 2.
(c) In ammonia (NH ), one atom of nitrogen combines with three atoms of hydrogen, thus the valency of
3
nitrogen is 3.
(d) In methane (CH ), one carbon atom combines with four atoms of hydrogen, thus the valency of carbon
4
is 4.
87