Page 105 - ICSE Chemistry 8
P. 105
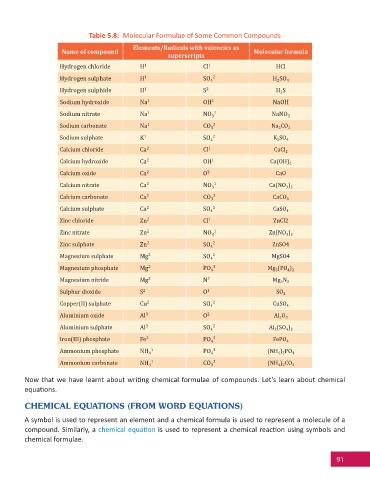
Table 5.8: Molecular Formulae of Some Common Compounds
Elements/Radicals with valencies as
Name of compound Molecular formula
superscripts
Hydrogen chloride H 1 Cl 1 HCl
Hydrogen sulphate H 1 SO 4 2 H SO 4
2
Hydrogen sulphide H 1 S 2 H S
2
Sodium hydroxide Na 1 OH 1 NaOH
Sodium nitrate Na 1 NO 3 1 NaNO 3
Sodium carbonate Na 1 CO 3 2 Na CO 3
2
Sodium sulphate K 1 SO 4 2 K SO 4
2
Calcium chloride Ca 2 Cl 1 CaCl 2
Calcium hydroxide Ca 2 OH 1 Ca(OH) 2
Calcium oxide Ca 2 O 2 CaO
Calcium nitrate Ca 2 NO 3 1 Ca(NO )
3 2
Calcium carbonate Ca 2 CO 3 2 CaCO 3
Calcium sulphate Ca 2 SO 4 2 CaSO 4
Zinc chloride Zn 2 Cl 1 ZnCl2
Zinc nitrate Zn 2 NO 3 1 Zn(NO )
3 2
Zinc sulphate Zn 2 SO 4 2 ZnSO4
Magnesium sulphate Mg 2 SO 4 2 MgSO4
Magnesium phosphate Mg 2 PO 4 3 Mg (PO )
3
4 2
Magnesium nitride Mg 2 N 3 Mg N 2
3
Sulphur dioxide S 2 O 1 SO 2
Copper(II) sulphate Cu 2 SO 4 2 CuSO 4
Aluminium oxide Al 3 O 2 Al 2 O 3
Aluminium sulphate Al 3 SO 4 2 Al 2 (SO 4 ) 3
Iron(III) phosphate Fe 3 PO 4 3 FePO 4
Ammonium phosphate NH 4 1 PO 4 3 (NH 4 ) 3 PO 4
Ammonium carbonate NH 4 1 CO 3 2 (NH 4 ) 2 CO 3
Now that we have learnt about wri ng chemical formulae of compounds. Let’s learn about chemical
equa ons.
CHEMICAL EQUATIONS (FROM WORD EQUATIONS)
A symbol is used to represent an element and a chemical formula is used to represent a molecule of a
compound. Similarly, a chemical equa on is used to represent a chemical reac on using symbols and
chemical formulae.
91