Page 108 - ICSE Chemistry 8
P. 108
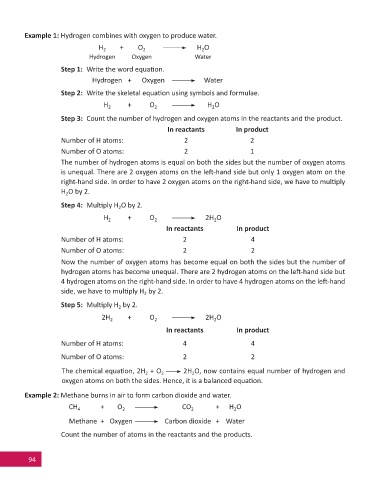
Example 1: Hydrogen combines with oxygen to produce water.
H 2 + O H O
2
2
Hydrogen Oxygen Water
Step 1: Write the word equa on.
Hydrogen + Oxygen Water
Step 2: Write the skeletal equa on using symbols and formulae.
H 2 + O H O
2
2
Step 3: Count the number of hydrogen and oxygen atoms in the reactants and the product.
In reactants In product
Number of H atoms: 2 2
Number of O atoms: 2 1
The number of hydrogen atoms is equal on both the sides but the number of oxygen atoms
is unequal. There are 2 oxygen atoms on the le -hand side but only 1 oxygen atom on the
right-hand side. In order to have 2 oxygen atoms on the right-hand side, we have to mul ply
H O by 2.
2
Step 4: Mul ply H O by 2.
2
H 2 + O 2H O
2
2
In reactants In product
Number of H atoms: 2 4
Number of O atoms: 2 2
Now the number of oxygen atoms has become equal on both the sides but the number of
hydrogen atoms has become unequal. There are 2 hydrogen atoms on the le -hand side but
4 hydrogen atoms on the right-hand side. In order to have 4 hydrogen atoms on the le -hand
side, we have to mul ply H by 2.
2
Step 5: Mul ply H by 2.
2
2H 2 + O 2H O
2
2
In reactants In product
Number of H atoms: 4 4
Number of O atoms: 2 2
The chemical equa on, 2H + O 2H O, now contains equal number of hydrogen and
2
2
2
oxygen atoms on both the sides. Hence, it is a balanced equa on.
Example 2: Methane burns in air to form carbon dioxide and water.
CH 4 + O CO 2 + H O
2
2
Methane + Oxygen Carbon dioxide + Water
Count the number of atoms in the reactants and the products.
94