Page 60 - ICSE Chemistry 8
P. 60
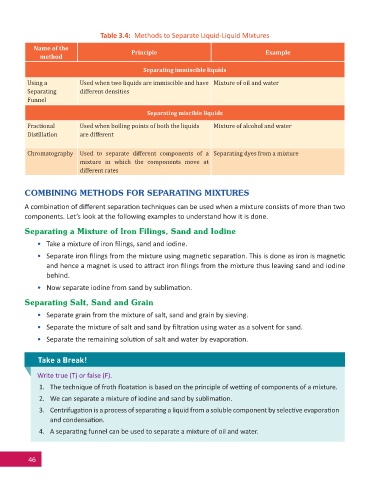
Table 3.4: Methods to Separate Liquid-Liquid Mixtures
Name of the Principle Example
method
Separating immiscible liquids
Using a Used when two liquids are immiscible and have Mixture of oil and water
Separating different densities
Funnel
Separating miscible liquids
Fractional Used when boiling points of both the liquids Mixture of alcohol and water
Distillation are different
Chromatography Used to separate different components of a Separating dyes from a mixture
mixture in which the components move at
different rates
COMBINING METHODS FOR SEPARATING MIXTURES
A combina on of diff erent separa on techniques can be used when a mixture consists of more than two
components. Let’s look at the following examples to understand how it is done.
Separating a Mixture of Iron Filings, Sand and Iodine
• Take a mixture of iron fi lings, sand and iodine.
• Separate iron fi lings from the mixture using magne c separa on. This is done as iron is magne c
and hence a magnet is used to a ract iron fi lings from the mixture thus leaving sand and iodine
behind.
• Now separate iodine from sand by sublima on.
Separating Salt, Sand and Grain
• Separate grain from the mixture of salt, sand and grain by sieving.
• Separate the mixture of salt and sand by fi ltra on using water as a solvent for sand.
• Separate the remaining solu on of salt and water by evapora on.
Take a Break!
Write true (T) or false (F).
1. The technique of froth fl oata on is based on the principle of we ng of components of a mixture.
2. We can separate a mixture of iodine and sand by sublima on.
3. Centrifuga on is a process of separa ng a liquid from a soluble component by selec ve evapora on
and condensa on.
4. A separa ng funnel can be used to separate a mixture of oil and water.
46