Page 58 - ICSE Chemistry 8
P. 58
Chromatography
The process of separa ng diff erent components of a mixture by passing it through a medium in which the
components move at diff erent rates is called chromatography. We can separate the components of ink
which is a mixture of diff erent dyes by this method.
The principle on which the process of chromatography is based is the diff erence in the rates of adsorp on
of various components of a mixture on the surface of a suitable adsorbent.
Filter paper and silica gel are commonly used adsorbents. The commonly used solvents are water, ace c
acid and ethyl alcohol.
Paper Chromatography
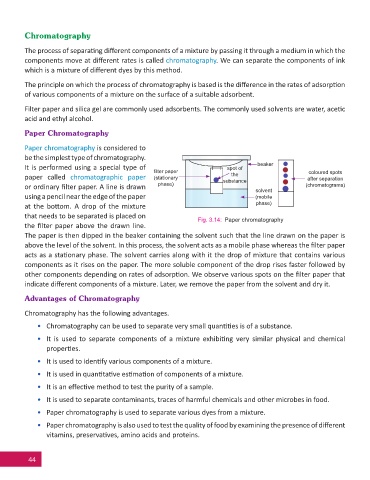
Paper chromatography is considered to
be the simplest type of chromatography.
beaker
It is performed using a special type of spot of
fi lter paper coloured spots
the
paper called chromatographic paper (stationary substance after separation
or ordinary fi lter paper. A line is drawn phase) (chromatograms)
solvent
using a pencil near the edge of the paper (mobile
phase)
at the bo om. A drop of the mixture
that needs to be separated is placed on
Fig. 3.14: Paper chromatography
the fi lter paper above the drawn line.
The paper is then dipped in the beaker containing the solvent such that the line drawn on the paper is
above the level of the solvent. In this process, the solvent acts as a mobile phase whereas the fi lter paper
acts as a sta onary phase. The solvent carries along with it the drop of mixture that contains various
components as it rises on the paper. The more soluble component of the drop rises faster followed by
other components depending on rates of adsorp on. We observe various spots on the fi lter paper that
indicate diff erent components of a mixture. Later, we remove the paper from the solvent and dry it.
Advantages of Chromatography
Chromatography has the following advantages.
• Chromatography can be used to separate very small quan es is of a substance.
• It is used to separate components of a mixture exhibi ng very similar physical and chemical
proper es.
• It is used to iden fy various components of a mixture.
• It is used in quan ta ve es ma on of components of a mixture.
• It is an eff ec ve method to test the purity of a sample.
• It is used to separate contaminants, traces of harmful chemicals and other microbes in food.
• Paper chromatography is used to separate various dyes from a mixture.
• Paper chromatography is also used to test the quality of food by examining the presence of diff erent
vitamins, preserva ves, amino acids and proteins.
44