Page 53 - ICSE Chemistry 8
P. 53
Observa on: Calcium carbonate (CaCO ) fi lters out. Some remains of CaCO are le partly in the beaker
3
3
and also on the fi lter paper. The fl akes of sodium chloride (NaCl) are found in the conical fl ask a er
evapora on.
Conclusion: This shows that CaCO and NaCl can be separated by fi ltra on and evapora on as CaCO is
3
3
insoluble whereas NaCl is soluble in water.
Fractional Crystallisation
Frac onal crystallisa on is the process used to separate components of a mixture when the solubility of
solid components varies in the same solvent.
We can separate a mixture of common salt and potassium nitrate by frac onal crystallisa on. Both the
components are soluble in water but the solubility of potassium nitrate is more as compared to the
solubility of sodium chloride in water. On cooling the solu on containing the mixture, potassium nitrate
crystallises and sodium chloride is le behind.
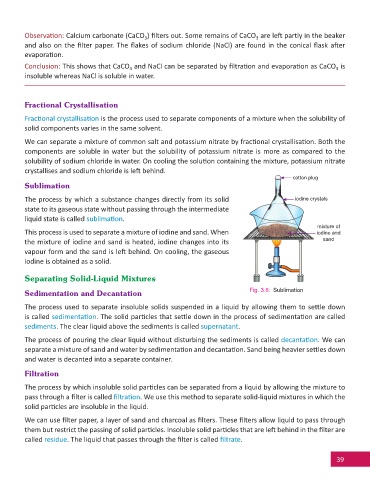
cotton plu
cotton plug
Sublimation
The process by which a substance changes directly from its solid iodine c
iodine crystalsr
state to its gaseous state without passing through the intermediate
liquid state is called sublima on.
mixture of
This process is used to separate a mixture of iodine and sand. When iodine and
the mixture of iodine and sand is heated, iodine changes into its sand
vapour form and the sand is le behind. On cooling, the gaseous
iodine is obtained as a solid.
Separating Solid-Liquid Mixtures
Sedimentation and Decantation Fig. 3.6: Sublimation
The process used to separate insoluble solids suspended in a liquid by allowing them to se le down
is called sedimenta on. The solid par cles that se le down in the process of sedimenta on are called
sediments. The clear liquid above the sediments is called supernatant.
The process of pouring the clear liquid without disturbing the sediments is called decanta on. We can
separate a mixture of sand and water by sedimenta on and decanta on. Sand being heavier se les down
and water is decanted into a separate container.
Filtration
The process by which insoluble solid par cles can be separated from a liquid by allowing the mixture to
pass through a fi lter is called fi ltra on. We use this method to separate solid-liquid mixtures in which the
solid par cles are insoluble in the liquid.
We can use fi lter paper, a layer of sand and charcoal as fi lters. These fi lters allow liquid to pass through
them but restrict the passing of solid par cles. Insoluble solid par cles that are le behind in the fi lter are
called residue. The liquid that passes through the fi lter is called fi ltrate.
39