Page 52 - ICSE Chemistry 8
P. 52
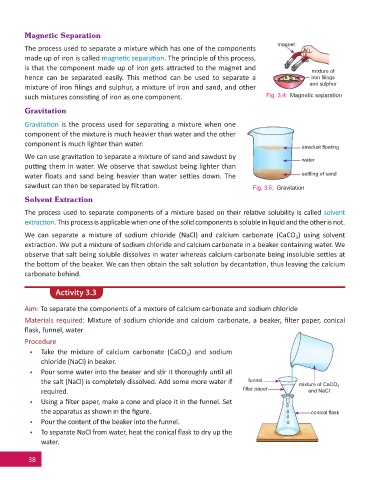
Magnetic Separation
magnet
The process used to separate a mixture which has one of the components
made up of iron is called magne c separa on. The principle of this process,
is that the component made up of iron gets a racted to the magnet and
mixture of
hence can be separated easily. This method can be used to separate a iron fi lings
and sulphur
mixture of iron fi lings and sulphur, a mixture of iron and sand, and other
such mixtures consis ng of iron as one component. Fig. 3.4: Magnetic separation
Gravitation
Gravita on is the process used for separa ng a mixture when one
component of the mixture is much heavier than water and the other
component is much lighter than water.
sawdust fl oating
We can use gravita on to separate a mixture of sand and sawdust by
water
pu ng them in water. We observe that sawdust being lighter than
water fl oats and sand being heavier than water se les down. The settling of sand
sawdust can then be separated by fi ltra on. Fig. 3.5: Gravitation
Solvent Extraction
The process used to separate components of a mixture based on their rela ve solubility is called solvent
extrac on. This process is applicable when one of the solid components is soluble in liquid and the other is not.
We can separate a mixture of sodium chloride (NaCl) and calcium carbonate (CaCO ) using solvent
3
extrac on. We put a mixture of sodium chloride and calcium carbonate in a beaker containing water. We
observe that salt being soluble dissolves in water whereas calcium carbonate being insoluble se les at
the bo om of the beaker. We can then obtain the salt solu on by decanta on, thus leaving the calcium
carbonate behind.
Activity 3.3
Aim: To separate the components of a mixture of calcium carbonate and sodium chloride
Materials required: Mixture of sodium chloride and calcium carbonate, a beaker, fi lter paper, conical
fl ask, funnel, water
Procedure
• Take the mixture of calcium carbonate (CaCO ) and sodium
3
chloride (NaCl) in beaker.
• Pour some water into the beaker and s r it thoroughly un l all
nel
the salt (NaCl) is completely dissolved. Add some more water if funnel mixture of CaCO
mixture of CaCO 3
paper
required. fi lter paper and Na
and NaClCl
• Using a fi lter paper, make a cone and place it in the funnel. Set
the apparatus as shown in the fi gure. co
conical fl asknical fl as
• Pour the content of the beaker into the funnel.
• To separate NaCl from water, heat the conical fl ask to dry up the
water.
38