Page 141 - ICSE Chemistry 8
P. 141
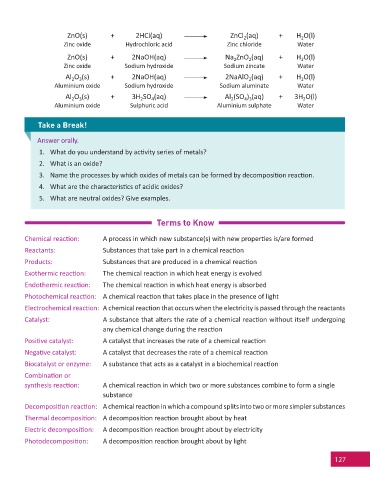
ZnO(s) + 2HCl(aq) ZnCl (aq) + H O(l)
2
2
Zinc oxide Hydrochloric acid Zinc chloride Water
ZnO(s) + 2NaOH(aq) Na ZnO (aq) + H O(l)
2
2
2
Zinc oxide Sodium hydroxide Sodium zincate Water
Al O (s) + 2NaOH(aq) 2NaAlO (aq) + H O(l)
3
2
2
2
Aluminium oxide Sodium hydroxide Sodium aluminate Water
Al O (s) + 3H SO (aq) Al (SO ) (aq) + 3H O(l)
2
2
4 3
2
3
2
4
Aluminium oxide Sulphuric acid Aluminium sulphate Water
Take a Break!
Answer orally.
1. What do you understand by ac vity series of metals?
2. What is an oxide?
3. Name the processes by which oxides of metals can be formed by decomposi on reac on.
4. What are the characteris cs of acidic oxides?
5. What are neutral oxides? Give examples.
Terms to Know
Chemical reac on: A process in which new substance(s) with new proper es is/are formed
Reactants: Substances that take part in a chemical reac on
Products: Substances that are produced in a chemical reac on
Exothermic reac on: The chemical reac on in which heat energy is evolved
Endothermic reac on: The chemical reac on in which heat energy is absorbed
Photochemical reac on: A chemical reac on that takes place in the presence of light
Electrochemical reac on: A chemical reac on that occurs when the electricity is passed through the reactants
Catalyst: A substance that alters the rate of a chemical reac on without itself undergoing
any chemical change during the reac on
Posi ve catalyst: A catalyst that increases the rate of a chemical reac on
Nega ve catalyst: A catalyst that decreases the rate of a chemical reac on
Biocatalyst or enzyme: A substance that acts as a catalyst in a biochemical reac on
Combina on or
synthesis reac on: A chemical reac on in which two or more substances combine to form a single
substance
Decomposi on reac on: A chemical reac on in which a compound splits into two or more simpler substances
Thermal decomposi on: A decomposi on reac on brought about by heat
Electric decomposi on: A decomposi on reac on brought about by electricity
Photodecomposi on: A decomposi on reac on brought about by light
127