Page 137 - ICSE Chemistry 8
P. 137
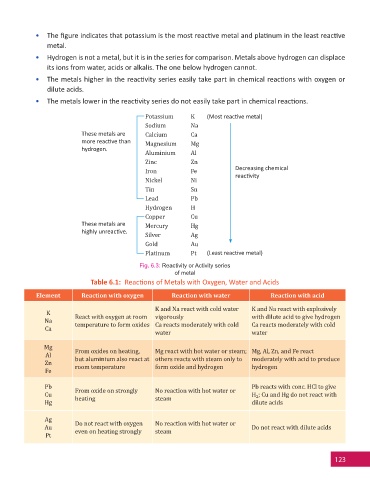
• The fi gure indicates that potassium is the most reac ve metal and pla num in the least reac ve
metal.
• Hydrogen is not a metal, but it is in the series for comparison. Metals above hydrogen can displace
its ions from water, acids or alkalis. The one below hydrogen cannot.
• The metals higher in the reac vity series easily take part in chemical reac ons with oxygen or
dilute acids.
• The metals lower in the reac vity series do not easily take part in chemical reac ons.
Potassium K (Most reac ve metal)
Sodium Na
These metals are Calcium Ca
more reac ve than Magnesium Mg
hydrogen.
Aluminium Al
Zinc Zn
Iron Fe Decreasing chemical
Nickel Ni reac vity
Tin Sn
Lead Pb
Hydrogen H
Copper Cu
These metals are Mercury Hg
highly unreac ve.
Silver Ag
Gold Au
Platinum Pt (Least reac ve metal)
Fig. 6.3: Reactivity or Activity series
of metal
Table 6.1: Reac ons of Metals with Oxygen, Water and Acids
Element Reaction with oxygen Reaction with water Reaction with acid
K and Na react with cold water K and Na react with explosively
K
Na React with oxygen at room vigorously with dilute acid to give hydrogen
Ca temperature to form oxides Ca reacts moderately with cold Ca reacts moderately with cold
water water
Mg From oxides on heating, Mg react with hot water or steam; Mg, Al, Zn, and Fe react
Al but aluminium also react at others reacts with steam only to moderately with acid to produce
Zn room temperature form oxide and hydrogen hydrogen
Fe
Pb From oxide on strongly No reaction with hot water or Pb reacts with conc. HCl to give
Cu heating steam H ; Cu and Hg do not react with
2
Hg dilute acids
Ag Do not react with oxygen No reaction with hot water or
Au even on heating strongly steam Do not react with dilute acids
Pt
123