Page 45 - ICSE Chemistry 6
P. 45
Observa on: You will no ce that a er some me the water starts
evapora ng from the beaker. A er some more me, all the water
evaporates and only salt is le behind.
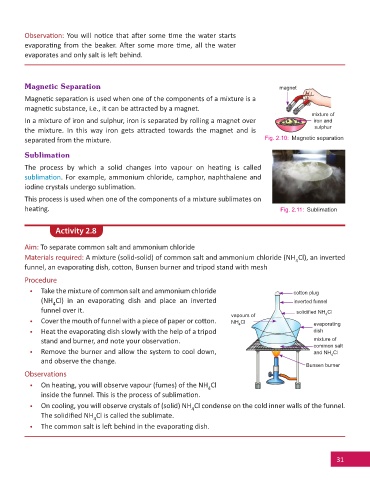
Magnetic Separation magnet
Magne c separa on is used when one of the components of a mixture is a
magne c substance, i.e., it can be a racted by a magnet.
mixture of
In a mixture of iron and sulphur, iron is separated by rolling a magnet over iron and
sulphur
the mixture. In this way iron gets a racted towards the magnet and is
separated from the mixture. Fig. 2.10: Magnetic separation
Sublimation
The process by which a solid changes into vapour on hea ng is called
sublima on. For example, ammonium chloride, camphor, naphthalene and
iodine crystals undergo sublima on.
This process is used when one of the components of a mixture sublimates on
hea ng. Fig. 2.11: Sublimation
Activity 2.8
Aim: To separate common salt and ammonium chloride
Materials required: A mixture (solid-solid) of common salt and ammonium chloride (NH Cl), an inverted
4
funnel, an evapora ng dish, co on, Bunsen burner and tripod stand with mesh
Procedure
• Take the mixture of common salt and ammonium chloride cotton plug
(NH Cl) in an evapora ng dish and place an inverted inverted funnel
4
funnel over it. solidifi ed NH Cl
vapours of 4
• Cover the mouth of funnel with a piece of paper or co on. NH Cl evaporating
4
• Heat the evapora ng dish slowly with the help of a tripod dish
stand and burner, and note your observa on. mixture of
common salt
• Remove the burner and allow the system to cool down, and NH Cl
4
and observe the change.
Bunsen burner
Observations
• On hea ng, you will observe vapour (fumes) of the NH Cl
4
inside the funnel. This is the process of sublima on.
• On cooling, you will observe crystals of (solid) NH Cl condense on the cold inner walls of the funnel.
4
The solidifi ed NH Cl is called the sublimate.
4
• The common salt is le behind in the evapora ng dish.
31