Page 44 - ICSE Chemistry 6
P. 44
Nature Care
The tea leaves and the fruit pulp that we fi lter in our houses every day are waste products that need to be
disposed off . Think and discuss eco-friendly and useful ways of their disposal.
Let’s understand fi ltra on by performing Ac vity 2.6.
Activity 2.6
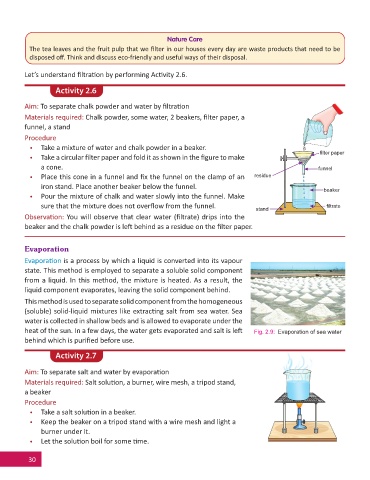
Aim: To separate chalk powder and water by fi ltra on
Materials required: Chalk powder, some water, 2 beakers, fi lter paper, a
funnel, a stand
Procedure
• Take a mixture of water and chalk powder in a beaker.
fi lter paper
• Take a circular fi lter paper and fold it as shown in the fi gure to make
a cone. funnel
• Place this cone in a funnel and fi x the funnel on the clamp of an residue
iron stand. Place another beaker below the funnel.
beaker
• Pour the mixture of chalk and water slowly into the funnel. Make
sure that the mixture does not overfl ow from the funnel. stand fi ltrate
Observa on: You will observe that clear water (fi ltrate) drips into the
beaker and the chalk powder is le behind as a residue on the fi lter paper.
Evaporation
Evapora on is a process by which a liquid is converted into its vapour
state. This method is employed to separate a soluble solid component
from a liquid. In this method, the mixture is heated. As a result, the
liquid component evaporates, leaving the solid component behind.
This method is used to separate solid component from the homogeneous
(soluble) solid-liquid mixtures like extrac ng salt from sea water. Sea
water is collected in shallow beds and is allowed to evaporate under the
heat of the sun. In a few days, the water gets evaporated and salt is le Fig. 2.9: Evaporation of sea water
behind which is purifi ed before use.
Activity 2.7
Aim: To separate salt and water by evapora on
Materials required: Salt solu on, a burner, wire mesh, a tripod stand,
a beaker
Procedure
• Take a salt solu on in a beaker.
• Keep the beaker on a tripod stand with a wire mesh and light a
burner under it.
• Let the solu on boil for some me.
30