Page 195 - ICSE Chemistry 8
P. 195
In the combined state, carbon occurs in the form of the following compounds.
• Carbon dioxide which is the main gaseous compound of
carbon Thirst for Knowledge
• Carbonates like chalk, limestone (CaCO ), marble, The earth’s crust contains only
3
dolomite (CaCO .MgCO ) and calamine (ZnCO ) very small amount of carbon
3
3
3
in the form of minerals like
• Fossil fuels like coal, petroleum and natural gas
carbonates, coal and petroleum.
• Organic compounds like carbo hydrates, fats and proteins
The atmosphere has only 0.03%
• Clothing material like co on, wool and silk of carbon dioxide gas.
COMPOUNDS OF CARBON
The number of compounds of carbon known at present is more than 5 million. Many more new carbon
compounds are being isolated or prepared in the laboratories everyday.
In fact, the number of carbon compounds alone is much more than the number of compounds of all other
elements taken together.
For the convenience of study, all the compounds are classifi ed into two classes—carbon compounds of
biological origin and carbon compounds of non-biological origin.
The compounds of carbon of biological origin are known as organic compounds. Such compounds
occur in all living things. Organic compounds also include
hydrocarbons and their deriva ves. Thirst for Knowledge
The compounds of carbon like oxides (carbon monoxide
The study of organic compounds such
and carbon dioxide), carbonates, hydrogen carbonates and
as hydrocarbons and their deriva ves
carbides come under the category of inorganic compounds
is called organic chemistry.
since they are of non-biological origin.
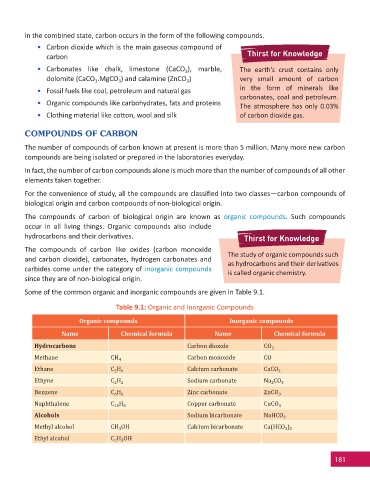
Some of the common organic and inorganic compounds are given in Table 9.1.
Table 9.1: Organic and Inorganic Compounds
Organic compounds Inorganic compounds
Name Chemical formula Name Chemical formula
Hydrocarbons Carbon dioxide CO 2
Methane CH 4 Carbon monoxide CO
Ethane C H 6 Calcium carbonate CaCO 3
2
Ethyne C H 2 Sodium carbonate Na CO 3
2
2
Benzene C H 6 Zinc carbonate ZnCO 3
6
Naphthalene C H 8 Copper carbonate CuCO 3
10
Alcohols Sodium bicarbonate NaHCO 3
Methyl alcohol CH OH Calcium bicarbonate Ca(HCO )
3 2
3
Ethyl alcohol C H OH
5
2
181