Page 37 - ICSE Chemistry 6
P. 37
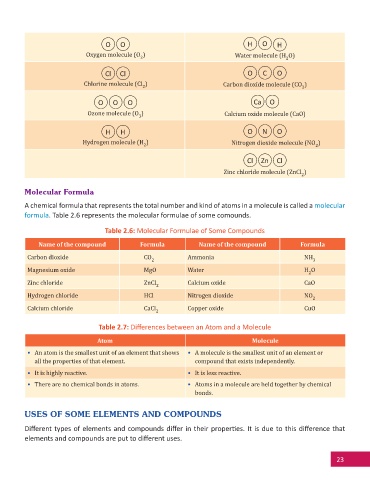
O O H O H
Oxygen molecule (O ) Water molecule (H O)
2 2
Cl Cl O C O
Chlorine molecule (Cl ) Carbon dioxide molecule (CO )
2 2
O O O Ca O
Ozone molecule (O ) Calcium oxide molecule (CaO)
3
H H O N O
Hydrogen molecule (H ) Nitrogen dioxide molecule (NO )
2 2
Cl Zn Cl
Zinc chloride molecule (ZnCl )
2
Molecular Formula
A chemical formula that represents the total number and kind of atoms in a molecule is called a molecular
formula. Table 2.6 represents the molecular formulae of some comounds.
Table 2.6: Molecular Formulae of Some Compounds
Name of the compound Formula Name of the compound Formula
Carbon dioxide CO 2 Ammonia NH 3
Magnesium oxide MgO Water H O
2
Zinc chloride ZnCl 2 Calcium oxide CaO
Hydrogen chloride HCl Nitrogen dioxide NO 2
Calcium chloride CaCl 2 Copper oxide CuO
Table 2.7: Diff erences between an Atom and a Molecule
Atom Molecule
• An atom is the smallest unit of an element that shows • A molecule is the smallest unit of an element or
all the properties of that element. compound that exists independently.
• It is highly reactive. • It is less reactive.
• There are no chemical bonds in atoms. • Atoms in a molecule are held together by chemical
bonds.
USES OF SOME ELEMENTS AND COMPOUNDS
Diff erent types of elements and compounds diff er in their proper es. It is due to this diff erence that
elements and compounds are put to diff erent uses.
23