Page 33 - ICSE Chemistry 6
P. 33
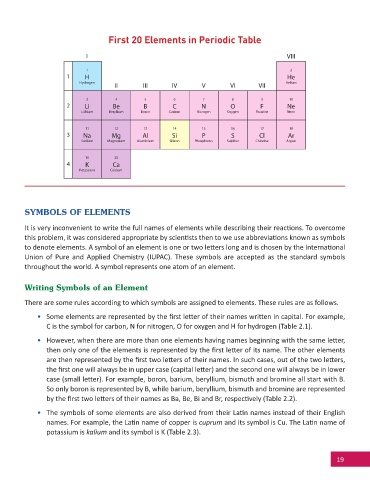
First 20 Elements in Periodic Table
I VIII
1 2
1 H He
Hydrogen Helium
II III IV V VI VII
3 4 5 6 7 8 9 10
2 Li Be B C N O F Ne
Lithium Beryllium Boron Carbon Nitrogen Oxygen Fluorine Neon
11 12 13 14 15 16 17 18
3 Na Mg Al Si P S Cl Ar
Sodium Magnesium Aluminium Silicon Phosphorus Sulphur Chlorine Argon
19 20
4 K Ca
Potassium Calcium
SYMBOLS OF ELEMENTS
It is very inconvenient to write the full names of elements while describing their reac ons. To overcome
this problem, it was considered appropriate by scien sts then to we use abbrevia ons known as symbols
to denote elements. A symbol of an element is one or two le ers long and is chosen by the Interna onal
Union of Pure and Applied Chemistry (IUPAC). These symbols are accepted as the standard symbols
throughout the world. A symbol represents one atom of an element.
Writing Symbols of an Element
There are some rules according to which symbols are assigned to elements. These rules are as follows.
• Some elements are represented by the fi rst le er of their names wri en in capital. For example,
C is the symbol for carbon, N for nitrogen, O for oxygen and H for hydrogen (Table 2.1).
• However, when there are more than one elements having names beginning with the same le er,
then only one of the elements is represented by the fi rst le er of its name. The other elements
are then represented by the fi rst two le ers of their names. In such cases, out of the two le ers,
the fi rst one will always be in upper case (capital le er) and the second one will always be in lower
case (small le er). For example, boron, barium, beryllium, bismuth and bromine all start with B.
So only boron is represented by B, while barium, beryllium, bismuth and bromine are represented
by the fi rst two le ers of their names as Ba, Be, Bi and Br, respec vely (Table 2.2).
• The symbols of some elements are also derived from their La n names instead of their English
names. For example, the La n name of copper is cuprum and its symbol is Cu. The La n name of
potassium is kalium and its symbol is K (Table 2.3).
19