Page 88 - Viva ICSE Science 4 : E-book
P. 88
TIME TO REVISE
1. Identify the solute, solvent and solution in a salt solution.
2. Are some gases soluble in water?
3. In a mixture of oil and water, which is lighter?
Methods of Separation of Soluble and Insoluble Substances
Substances in a solution can be separated using simple separation methods. The method
of single separation depends on the type of solution. As the insoluble substances do
not dissolve in the solvent, they can be separated by sedimentation, decantation and
fi ltration methods. The soluble substances can be separated by evaporation method.
The methods of separation are mentioned below.
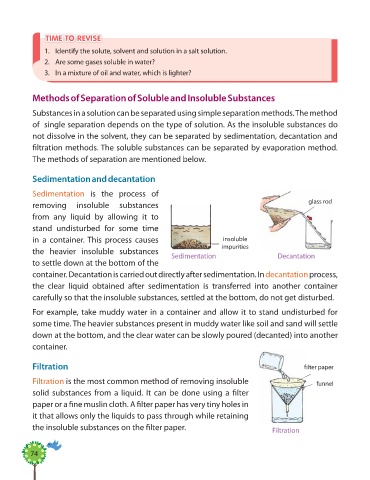
Sedimentation and decantation
Sedimentation is the process of
glass rod
removing insoluble substances
from any liquid by allowing it to
stand undisturbed for some time
in a container. This process causes insoluble
impurities
the heavier insoluble substances
Sedimentation Decantation
to settle down at the bottom of the
container. Decantation is carried out directly after sedimentation. In decantation process,
the clear liquid obtained after sedimentation is transferred into another container
carefully so that the insoluble substances, settled at the bottom, do not get disturbed.
For example, take muddy water in a container and allow it to stand undisturbed for
some time. The heavier substances present in muddy water like soil and sand will settle
down at the bottom, and the clear water can be slowly poured (decanted) into another
container.
Filtration f ilter paper
Filtration is the most common method of removing insoluble funnel
solid substances from a liquid. It can be done using a fi lter
paper or a fi ne muslin cloth. A fi lter paper has very tiny holes in
it that allows only the liquids to pass through while retaining
the insoluble substances on the fi lter paper. Filtration
74